Structure of Hexamethylene Triperoxide Diamine. By William P. Schaefer,
John T. Forukas, and Bruce G. Tiemann. Received November 2, 1984.
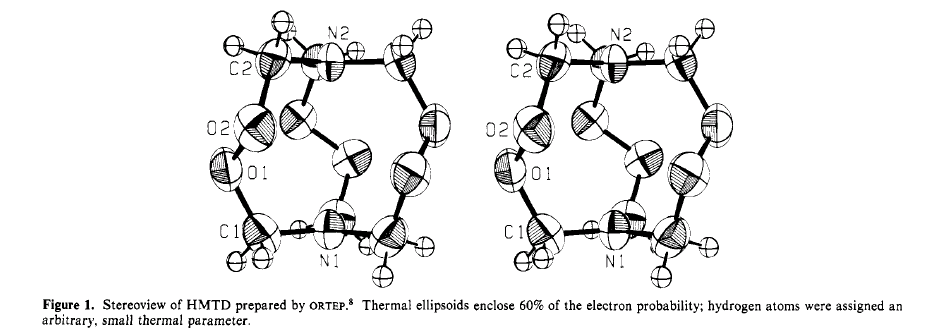
1,6-Diaza-3,4,8,9,12,13-hexaoxabicyclo[4,4,4]tetradecane (hexamethylene
triperoxide diamine or HMTD) was first synthesized in 1885 by Legler (Legler,
L. Ber. 1885, 18, 3343-3351.) It was discovered to be
a powerful initiating explosive and was studied in this capacity by Taylor
and Rinkenbach of the Bureau of Mines (Taylor, C. A.; Rinkenbach, W. H. Army
Ordnance 1924. 5, 463-466.) It has advantages over many
primary explosives. It is relatively insensitive to shock, (requires a 3
cm drop of a 2 kg weight to detonate, compared with a 0.25 cm drop for mercury
fulminate), more powerful than most initiating explosives, inexpensive, and
easy to synthesize. It decomposes slowly in storage and is thus not in military
or commercial use. "We were first attracted to HMTD by a stick model of
the molecule that suggested that there might be a central cavity toward
which some lone pair electrons from the oxygen atoms were pointing (Figure
1). ... We began a crystallographic study to determine the size and configuration
of the cavity; our results are reported in this paper."
Experimental:
Fourteen grams of hexamethylenetetramine is dissolved in 45 g of 30%
hydrogen peroxide and stirred mechanically at 0 degrees. Twenty-one grams
of powdered citric acid is slowly added with continued stirring. The mixture
is stirred for 3 hours at 0 degrees and then allowed to warm to room temperature
and stand for 2 hours. The white crystalline product is filtered off, thoroughly
washed with water, rinsed with methanol, and air dried. If more than double
the recommended amount is made at once, the product will decompose exothermally
while the solution warms. HMTD may be stored under water with no explosion
risk and no increase in the rate of decomposition.
A crystal (0.16 x 0.19 x 0.21 mm) that appeared satisfactory was centered
on a CAD-4 diffractometer and a rhombohedral cell was found. [much writing
of interest to crystallographers but beyond the experimental equipment
means of Mad Scientists discarded]
Results and Discussion:
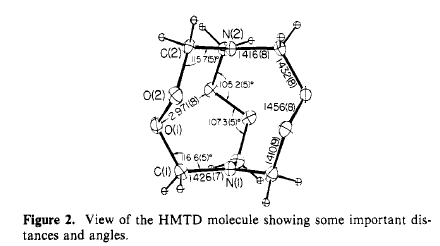
The 2 nitrogen atoms have an unusual geometry: each is planar within
experimental error. N(1) is only 0.006 angstroms out of the plane of the
C(1) atoms, while N(2) is 0.025 angstroms from the C(2) plane. The angles
C-N-C are all 120.0 degrees. A normal tetrahedral nitrogen atom with N-C
bonds of 1.47 angstroms would be 0.49 angstroms out of the carbon atom plane.
The largest difference between HMTD and comparable compounds is found
in the torsion angle C-O-O-C. In HMTD this angle is 130 degrees, 30 degrees
larger than expected in the absence of other effects. A smaller angle would
decrease the nonbonded O(1)-O(2) distance and force the two nitrogens closer
together, both of which are unfavorable. In HMTD there is also an electrostatic
repulsion between the nitrogen and oxygen that tends to keep the torsion
angle large.
"Electronic effects must represent some balance between the loss in energy
caused by the sp2 hybridization of the nitrogen atoms (rather
than sp3) and the gain obtained by shorter C-N bonds. Since isolated
C3N systems are not planar, this balance must be net negative,
but is more than compensated for by the other factors. ... The fact that
this planar arrangement has been observed once before, in the macrobicyclic
cryptand (Newkome, G. R.;Majestic, V.; Fronczek, F. R.; Atwood, J. L. J.
Am. Chem. Soc. 1979, 101, 1047-1048), leads us to believe that
it is a stable electronic arrangement for an attached C3N fragment."
"There is still the question of inserting a proton in the central cavity
of HMTD. ... Evidently direct protonation is not effective; the material
was synthesized in acidic solution, but no evidence for a proton between
the nitrogen atoms can be found in the electron density maps. Thus, although
the cavity appears to be large enough to contain a proton, because we have
not been able to protonate it we infer that the oxygen atoms protect the
cavity well."
The preceding information was taken from an article in J. Am. Chem.
Soc., Vol. 107, No. 8, 1985, and condensed/edited by Polverone.

